Artificial Intelligence-based agents in chronic liver disease: transforming diagnostic and therapeutic workflows through clinical decision-making

Chronic liver diseases encompass a complex continuum spanning metabolic dysfunction-associated steatotic liver disease (MASLD), steatohepatitis (MASH), alcohol-related liver disease (ALD), metabolic-ALD (met-ALD), primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), and their malignant transformations including hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA)1. These conditions share pathophysiological pathways involving hepatic inflammation, metabolic dysregulation, and progressive fibrosis, yet present unique diagnostic and therapeutic challenges requiring sophisticated clinical coordination.
MASLD affects approximately 25% of the global population, surpassing viral hepatitis as the leading chronic liver disease worldwide2. The complex interplay between metabolic and alcohol factors has established met-ALD as a distinct clinical entity separate from pure ALD, with unique prognostic implications3,4. Similarly, cholestatic diseases including PSC and PBC represent distinct autoimmune conditions with different pathophysiological mechanisms and surveillance requirements. Cholestatic diseases, while less prevalent, present particularly challenging scenarios, with PSC patients carrying a 400-fold increased CCA risk compared to the general population5. Traditional clinical workflows necessitate sequential coordination between multiple specialists radiologists, hepatologists, interventional radiologists, pathologists, and molecular geneticists each contributing isolated assessments requiring manual integration and clinical synthesis.
Unlike conventional artificial intelligence systems that operate as static databases or isolated analytical tools, AI agents represent autonomous software systems capable of reasoning, planning, and executing complex clinical tasks through continuous cycles of assessment, adaptation, and collaboration6. These systems form dynamic collaborative networks where individual agents communicate, negotiate solutions, and redistribute computational tasks based on case complexity. True multi-agent systems emerge when agents establish communication protocols enabling distributed problem-solving, where collective intelligence exceeds individual agent capabilities.
AI agents could demonstrate transformative potential for autonomous imaging triage, real-time clinical data integration, dynamic treatment selection, and continuous disease monitoring across the chronic liver disease spectrum. Through standardized inter-agent communication protocols, these systems would enable collaborative reasoning where multiple specialized agents contribute domain expertise to complex diagnostic challenges. When diagnostic uncertainty arises, agents engage in structured deliberation protocols, sharing evidence with quantified confidence scores until consensus emerges or appropriate escalation to human specialists occurs.
Chronic liver disease management challenges
Chronic liver diseases present multifaceted diagnostic and therapeutic challenges that exemplify the need for coordinated clinical decision-making. These conditions share overlapping pathophysiological mechanisms while requiring disease-specific expertise, making them ideal candidates for AI agent coordination systems. Notably, MetALD and ALD represent distinct diagnostic categories rather than progressive stages, as do PSC and PBC among cholestatic diseases (Table 1) (Fig.1).
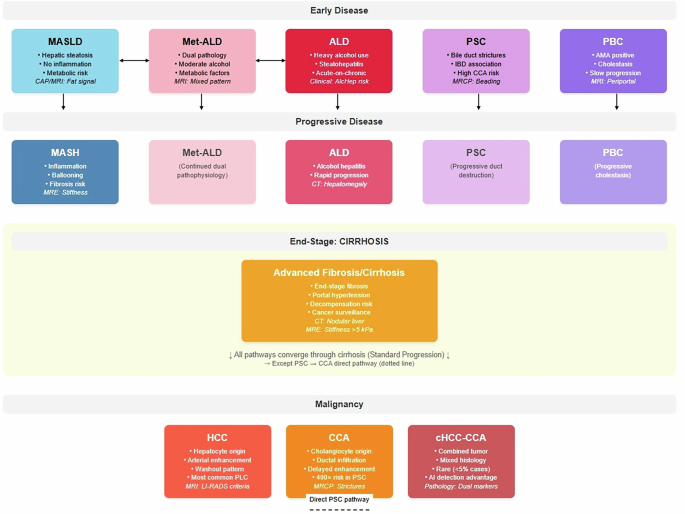
Comprehensive disease progression model demonstrating parallel and interconnected pathways of chronic liver diseases. The metabolic pathway (MASLD → MASH, blue) represents steatosis-to-steatohepatitis progression driven by obesity, diabetes, and metabolic dysfunction. MetALD (pink) and ALD (red) are shown as separate parallel conditions rather than sequential progression, reflecting current nomenclature where MetALD represents concurrent metabolic and alcohol-related factors with moderate alcohol consumption, while ALD involves heavy alcohol use with acute-on-chronic hepatitis risk. These conditions may transition bidirectionally based on drinking pattern changes but represent distinct diagnostic entities. Cholestatic diseases (PSC and PBC, purple shades) are presented as separate parallel autoimmune conditions, each with distinct pathophysiology PSC characterized by bile duct strictures and IBD association, PBC by AMA-positive status and slower progression. PSC carries exceptional malignant transformation risk (400-fold increased CCA risk). All pathways converge through cirrhosis (orange) to primary liver cancers, with the critical exception that PSC demonstrates direct cholangiocarcinoma development (dashed arrow) independent of cirrhosis. Malignant endpoints include hepatocellular carcinoma (HCC, arising from hepatocytes), cholangiocarcinoma (CCA, from cholangiocytes), and rare combined tumors (cHCC-CCA, <5% of cases) exhibiting mixed histology detectable through AI pathology. Molecular stratification spans metabolic risk genes (PNPLA3, TM6SF2), HCC drivers (TP53, CTNNB1), and CCA mutations (IDH1/2, FGFR2), enabling precision surveillance and therapeutic targeting. Clinical significance: Solid arrows indicate standard cirrhosis-mediated progression; dashed arrow represents direct malignant risk pathway. Color coding facilitates pathway recognition: blue (metabolic), pink (Met-ALD), red (alcohol-related), purple (cholestatic), orange (end-stage), dark red (malignant) supporting multidisciplinary care coordination and risk stratification across the chronic liver disease spectrum.
link