Liver Disease Diagnostics Market | Global Market Analysis Report
Liver Disease Diagnostics Market Forecast and Outlook 2026 to 2036
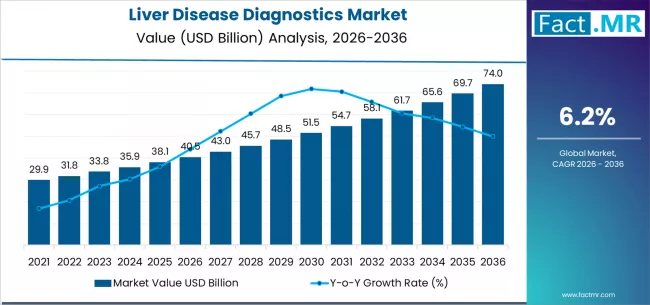
The global liver disease diagnostics market is projected to grow from USD 40.46 billion in 2026 to USD 74.0 billion by 2036. This translates into a CAGR of 6.2% between 2026 and 2036. The market is expected to grow by over 1.8X during this period, supported by exponential demand for non-invasive diagnostic solutions, rising adoption of advanced imaging technologies, and growing emphasis on early disease detection innovation and patient outcome optimization across global hepatology healthcare operations.
Key Takeaways from the Liver Disease Diagnostics Market
- Liver Disease Diagnostics Market Value (2026): USD 40.46 billion
- Liver Disease Diagnostics Market Forecast Value (2036): USD 74.0 billion
- Liver Disease Diagnostics Market Forecast CAGR (2026 to 2036): 6.2%
- Leading Diagnosis Technique in Liver Disease Diagnostics Market: Imaging (30.6%)
- Leading Disease in Liver Disease Diagnostics Market: NAFLD (26.4%)
- Leading End Use in Liver Disease Diagnostics Market: Hospitals (44.6%)
- Key Growth Regions in Liver Disease Diagnostics Market: Asia Pacific, North America, and Europe
- Key Players in Liver Disease Diagnostics Market: Abbott, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Randox Laboratories Ltd., Boston Scientific Corporation, Laboratory Corporation of America Holdings, Fujifilm Corporation, Horiba Medical, Siemens Healthcare GmbH, Quest Diagnostics

The liver disease diagnostics market is positioned for substantial expansion, driven by increasing recognition of NAFLD epidemic importance, growing prevalence of metabolic liver diseases with enhanced screening standards, and rising adoption of advanced molecular diagnostic platforms across hepatology and gastroenterology practices globally.
The market demonstrates robust fundamentals supported by expanding specialty diagnostic laboratory networks, healthcare professionals’ focus on non-invasive fibrosis assessment protocols, and rising recognition of liver disease screening as critical healthcare components in achieving enhanced early detection, disease staging capabilities, and treatment monitoring effectiveness within modern healthcare architectures across diverse patient populations.
Liver Disease Diagnostics Market
| Metric | Value |
|---|---|
| Estimated Value (2026E) | USD 40.46 billion |
| Forecast Value (2036F) | USD 74.0 billion |
| Forecast CAGR (2026 to 2036) | 6.2% |
Category
| Category | Segments |
|---|---|
| Diagnosis Technique | Laboratory Tests; Imaging; Endoscopy; Biopsy; Others |
| Disease | NAFLD; NASH; Fibrosis; Cirrhosis; Hepatocellular Carcinoma (HCC); Others |
| End Use | Hospitals; Laboratories; Others |
| Region | Asia Pacific; North America; Europe; Latin America; MEA |
Segmental Analysis
How Does Imaging Technique Drive Market Leadership in Liver Disease Diagnostics?
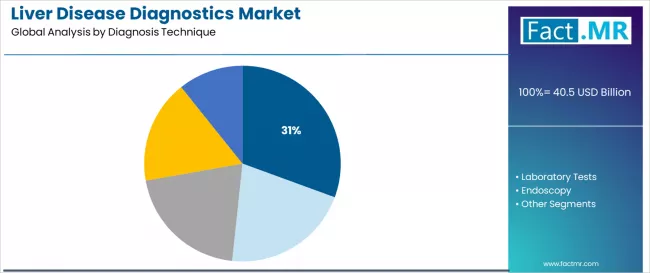
Imaging diagnostics is projected to account for 30.6% of the liver disease diagnostics market in 2026, reaffirming its position as the category’s dominant diagnostic methodology specification. Healthcare providers increasingly recognize the optimal balance of non-invasive assessment and comprehensive disease characterization offered by imaging-based diagnostics for liver disease evaluation, particularly in elastography applications and cross-sectional imaging environments.
This technique category addresses both fibrosis staging requirements and anatomical assessment demands while providing patient-friendly diagnostic options across diverse clinical populations.
Why does NAFLD Command Majority Disease Market Share?
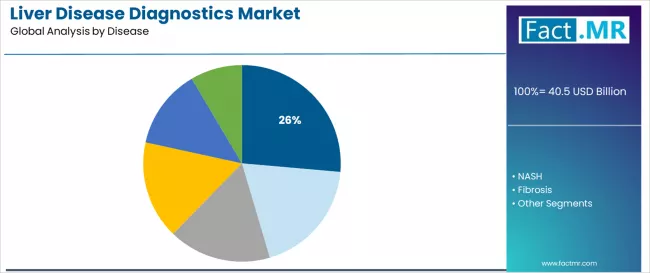
NAFLD disease category is projected to represent 26.4% of liver disease diagnostics demand in 2026, underscoring its role as the primary disease driver reflecting epidemic prevalence and screening requirements. Healthcare providers recognize that NAFLD assessment, including diverse disease stages, metabolic risk factors, and progression monitoring protocols, provides the largest diagnostic volume that other liver diseases cannot match in population prevalence and screening demand. NAFLD diagnostics utilized in metabolic health programs offer comprehensive risk assessment and disease staging essential for serving primary care screening and hepatology referral requirements.
Why are Hospitals the Preferred Sites for Liver Disease Diagnostics?
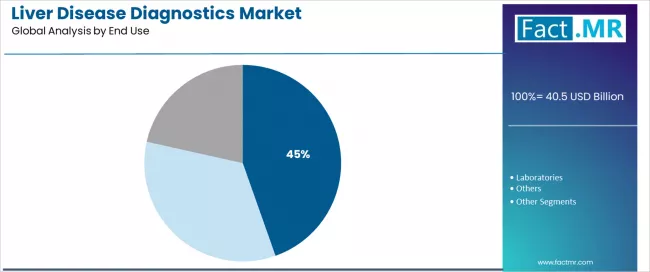
The hospital end use segment is projected to account for 44.6% of the liver disease diagnostics market in 2026, establishing its position as the leading care setting segment. Healthcare systems increasingly recognize that hospital-based diagnostics, encompassing inpatient assessment, emergency department evaluation, and outpatient specialty clinics, represent the most clinically integrated and comprehensive diagnostic environment for liver disease management due to multimodality access and subspecialty consultation availability. This end use type addresses both acute liver disease presentation and chronic disease monitoring requirements while delivering coordinated diagnostic services across specialized care teams.
What are the Drivers, Restraints, and Key Trends of the Liver Disease Diagnostics Market?
The liver disease diagnostics market is driven by the rising prevalence of nonalcoholic fatty liver disease (NAFLD) and increasing demand for non-invasive diagnostic solutions across hepatology care. Growing clinical focus on early detection, disease staging, and longitudinal monitoring is supporting adoption of advanced diagnostic tools. Technological progress in imaging and data analytics is further strengthening diagnostic capabilities and clinical confidence.
Market restraints include limited diagnostic sensitivity in early-stage disease, which can delay detection and intervention. High costs of advanced imaging technologies restrict access in resource-limited healthcare settings. Lack of standardization across diagnostic platforms also creates variability in results and clinical interpretation.
Key trends include expanding use of elastography-based technologies and multiparametric assessment systems that enable quantitative fibrosis measurement and improved NASH characterization. Integration of artificial intelligence and digital diagnostic platforms is also accelerating, supporting automated image analysis, disease progression prediction, and clinical decision support. These developments are improving diagnostic consistency, risk stratification, and personalized disease management while enhancing efficiency across healthcare systems.
Analysis of the Liver Disease Diagnostics Market by Key Countries
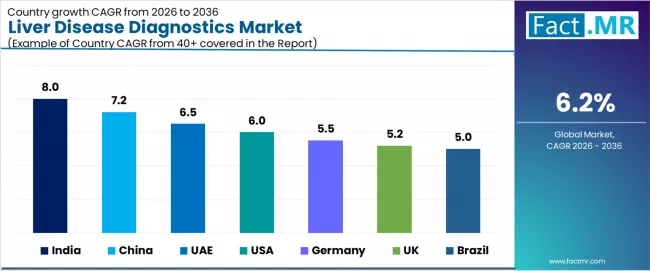
| Country | CAGR (2026-2036) |
|---|---|
| India | 8.0% |
| China | 7.2% |
| UAE | 6.5% |
| USA | 6.0% |
| Germany | 5.5% |
| UK | 5.2% |
| Brazil | 5.0% |
Why is India Emerging as a High-Growth Market for Liver Disease Diagnostics?
India’s liver disease diagnostics market is expanding at a CAGR of 8.0% through 2036, largely due to the sharp rise in NAFLD and other metabolic liver conditions. Urban lifestyles, increasing obesity, and diabetes prevalence are pushing liver screening into mainstream clinical practice.
Growth is strongest in private hospitals and large diagnostic chains, where non-invasive tools such as elastography and biomarker-based tests are gaining preference for early detection. Rising middle-class health awareness and government focus on non-communicable diseases are reinforcing demand for accessible, cost-effective diagnostics.
How is China Driving Scale-Led Growth in Liver Disease Testing?
China’s market is growing at a 7.2% CAGR, supported by a substantial burden of both viral hepatitis and metabolic liver disease. Large patient volumes and expanding tertiary care infrastructure are accelerating adoption of non-invasive diagnostics across hospitals and screening programs.
Investments in elastography systems and advanced laboratory platforms are improving early diagnosis, while government-backed screening initiatives are widening access. Growth reflects both disease prevalence and the healthcare system’s push toward earlier intervention and standardized assessment.
What is powering the UAE’s Expansion in Liver Disease Diagnostics?
The UAE is seeing steady growth at a 6.5% CAGR, driven by healthcare modernization and rising demand for preventive screening. Specialty gastroenterology centers and premium hospitals are adopting advanced diagnostic tools to support metabolic disease management.
Medical tourism and government health initiatives are encouraging early detection, with a strong emphasis on accuracy and international care standards. Liver diagnostics are increasingly positioned as part of routine preventive health checks rather than reactive testing.
Why does the USA Remain a Technology-Driven Diagnostics Market?
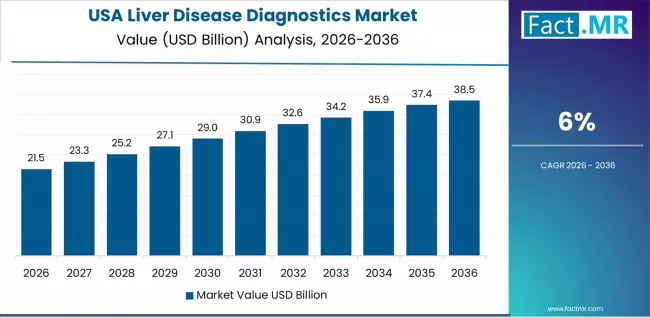
The USA market is advancing at a 6.0% CAGR, shaped by widespread adoption of non-invasive liver assessment and strong clinical evidence supporting early NAFLD detection. Academic centers and community practices alike are integrating elastography with biomarker panels to improve disease staging. Guideline-driven screening, reimbursement support, and continuous clinical research reinforce growth, making liver diagnostics a routine part of metabolic and hepatology care.
How is Germany Sustaining Growth Through Healthcare System Strength?
Germany’s liver disease diagnostics market is growing at a 5.5% CAGR, anchored in a robust healthcare system and strict clinical standards. Hospitals and specialist practices rely heavily on validated diagnostic technologies that meet regulatory and guideline requirements.
Growth is steady rather than rapid, reflecting systematic screening, strong physician adherence to evidence-based protocols, and consistent investment in quality-assured diagnostic platforms.
What is Driving Preventive Liver Screening Adoption in the UK?
The UK market is expanding at a 5.2% CAGR, supported by preventive healthcare priorities and NHS-led screening pathways. Liver diagnostics are increasingly used in primary care to identify high-risk patients early, particularly those with metabolic conditions.
Cost-effectiveness and population-level impact shape technology adoption, with non-invasive tests fitting well into national screening strategies and resource-conscious healthcare planning.
How is Brazil Expanding Access through Diagnostic Network Growth?
Brazil’s liver disease diagnostics market is growing at a 5.0% CAGR, driven by the expansion of private laboratory networks and improving urban healthcare access. Diagnostic providers are focusing on making liver testing more widely available, especially in metropolitan areas. Growth reflects increasing disease awareness and gradual adoption of non-invasive technologies that balance clinical value with affordability.
Competitive Landscape of the Liver Disease Diagnostics Market
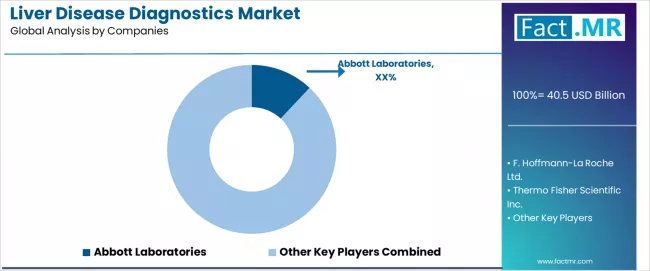
The liver disease diagnostics market is highly competitive, with participation from global diagnostics manufacturers, medical device companies, and integrated laboratory service providers. Competition is driven by diagnostic accuracy, clinical validation strength, system integration, and accessibility across healthcare settings rather than price-based factors. Ongoing investment in biomarker innovation, non-invasive testing technologies, and healthcare provider partnerships underpins market positioning.
Abbott Laboratories holds a leading position, supported by point-of-care testing capabilities and established biomarker-based diagnostic platforms used across hospital and outpatient settings. F. Hoffmann-La Roche Ltd. competes through automated laboratory systems and molecular diagnostics integrated into large clinical laboratories. Thermo Fisher Scientific Inc. maintains a strong presence through analytical instruments and reagent portfolios serving both research and clinical diagnostics. Randox Laboratories Ltd. differentiates through specialized biomarker panels and automated immunoassay solutions for liver function assessment.
Other participants include Boston Scientific Corporation and Fujifilm Corporation, which support hepatology diagnostics through endoscopy and imaging technologies. Laboratory Corporation of America Holdings and Quest Diagnostics strengthen competition through broad test menus and nationwide laboratory networks, while Siemens Healthcare GmbH and Horiba Medical emphasize integrated diagnostic systems and laboratory efficiency. The competitiveness overall favors companies combining validated diagnostics, scalable platforms, and strong healthcare integration.
Key Players in the Liver Disease Diagnostics Market
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- Randox Laboratories Ltd.
- Boston Scientific Corporation
- Laboratory Corporation of America Holdings
- Fujifilm Corporation
- Horiba Medical
- Siemens Healthcare GmbH
- Quest Diagnostics
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2026) | USD 40.46 Billion |
| Diagnosis Technique | Laboratory Tests, Imaging, Endoscopy, Biopsy, Others |
| Disease | NAFLD, NASH, Fibrosis, Cirrhosis, HCC, Others |
| End Use | Hospitals, Laboratories, Others |
| Regions Covered | Asia Pacific, North America, Europe, Latin America, MEA |
| Countries Covered | USA, Germany, UK, China, India, UAE, Brazil and 40+ countries |
| Key Companies Profiled | Abbott, F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Randox Laboratories Ltd., Boston Scientific Corporation, Laboratory Corporation of America Holdings, Fujifilm Corporation, Horiba Medical, Siemens Healthcare GmbH, Quest Diagnostics |
| Additional Attributes | Dollar sales by diagnosis technique, disease, end use, regional demand trends, competitive landscape, healthcare provider preferences for specific liver disease diagnostics, integration with comprehensive hepatology care pathways, innovations in non-invasive assessment development, imaging technology advancement, and clinical validation optimization capabilities |
Liver Disease Diagnostics Market by Segments
-
Diagnosis Technique :
- Laboratory Tests
- Imaging
- Endoscopy
- Biopsy
- Others
-
Disease :
- NAFLD
- NASH
- Fibrosis
- Cirrhosis
- HCC
- Others
-
End Use :
- Hospitals
- Laboratories
- Others
-
Region :
-
Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
North America
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
-
MEA
- Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of MEA
-
link

:max_bytes(150000):strip_icc()/GettyImages-2214947723-0a716689cecf44e0b2c65b431b708386.jpg)


